This paper discusses the conditions under which duplex can suffer chloride SCC and describes some service successes and failures.
By Roger Francis, Nickel Institute
It is well known that the 300 series austenitic stainless steels, such as 316L (UNS S31603), are susceptible to chloride stress corrosion cracking (SCC) at low concentrations of chloride and at temperatures exceeding 55°C1. This has caused numerous service failures, as described, for example, in reference 1. Duplex stainless steels are well known to have good resistance to chloride SCC, and there is data that suggests the chloride and temperature limits for different grades of duplex stainless steels1. What is less well known is that failures can occur within these limits under certain environmental conditions.
Standard tests
Note: the nominal compositions of the alloys discussed in this paper and their UNS numbers are shown in Table 1.
There are a number of standard tests for SCC, and some modifications of them, such as the ASTM G36 boiling magnesium chloride test2 and the G123 test in boiling acidified sodium chloride3. Table 2 presents the results of tests on some austenitic and duplex stainless steels4-6. It can be seen that everything fails in the G36 test, and it is just the time to failure that varies.
In the other tests, the austenitic alloys fail, and the duplex alloys pass, which doesn’t help in assessing alloys for SCC resistance in a specific fluid unless it is the one in the test.
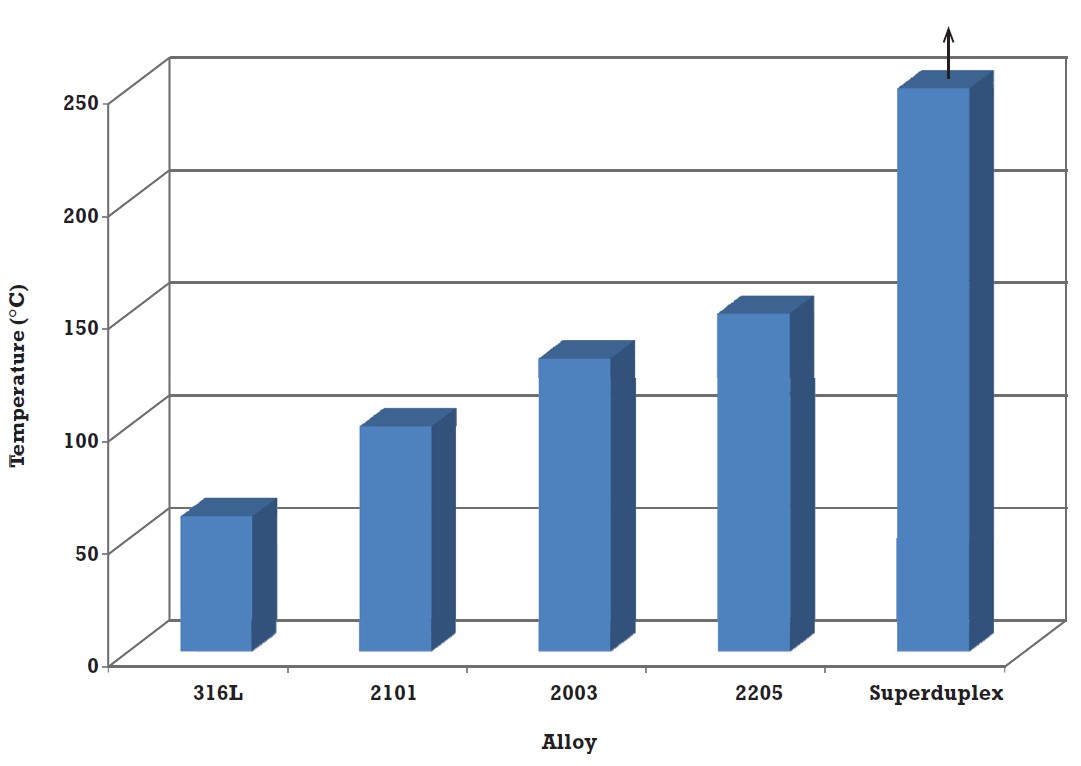
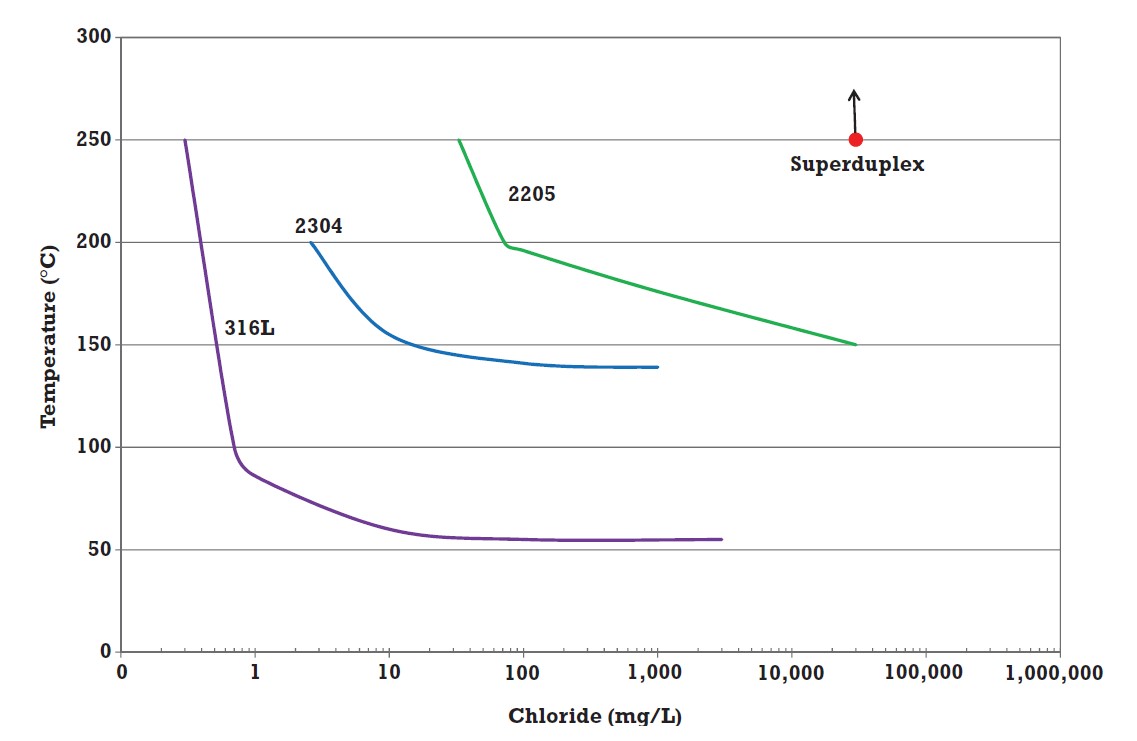
Another test used to compare the SCC resistance of stainless steels is immersion in aerated 5% sodium chloride at around the 0.2% proof stress and determining the temperature at which SCC initiates. The results for some alloys are shown in Figure 1, and while this is a little more useful than the data in Table 2, it doesn’t help very much with alloy selection7. By doing a lot of tests in solutions with different chloride contents and at different temperatures, it is possible to produce curves such as those in Figure 2. The data were generated in pure aerated solution, making the conditions mildly oxidising, and this has proved useful for many applications. However, there are service environments where the conditions are strongly oxidising or strongly reducing.
Redox potential
Figure 3 shows the typical polarisation curve for stainless steel in a corrosive solution. When it resists corrosion it is in the passive zone, but if the conditions become more reducing, the potential can decrease, and the alloy moves into the active zone, where general dissolution occurs. Under oxidising conditions the alloy can move into the transpassive zone, where increased dissolution may occur, or pitting if chlorides are present. The redox potential indicates whether the conditions are reducing, oxidising or neutral. SCC is possible at the transition zones between active and passive, and passive and transpassive.
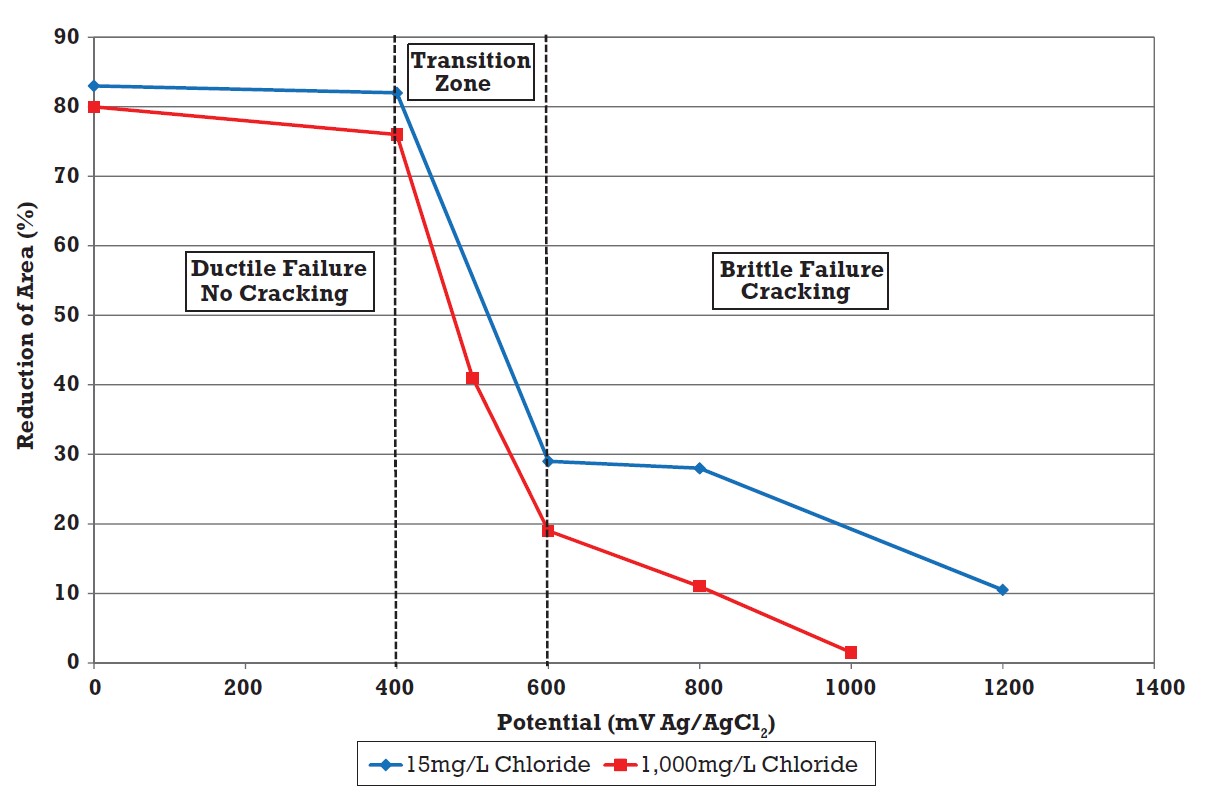
Atkinson et al carried out slow strain rate tests on S32760 super duplex stainless steel in aerated solutions at 130°C, and chloride concentrations from 15 to 1,000 mg/L8. The results in Figure 4 show that at lower potentials the alloy had good resistance to cracking, while at potentials ≥ 600 mV SCE the alloy was very susceptible to cracking. From 400 mV to 600 mV SCE there was a transition region where the ductility decreased as the potential increased. These results clearly demonstrated that under oxidising conditions, the alloy was susceptible to SCC at chloride concentrations as low as 15 mg/L, at 130°C.
The potentials of stainless steels are not reported for many fluids, but they are known for seawater at ambient temperature9. If a biofilm forms, the potential is around 300 mV SCE, and the conditions are moderately oxidising. If chlorine or hypochlorite is injected to prevent the formation of a biofilm (~ 0.5 mg/L), the potential rises to around 600 mV SCE, and the conditions are very oxidising. If the oxygen is removed from the seawater, the potential is ~ -100 mV SCE with 200 ppb oxygen, which is roughly neutral. More negative potentials can indicate reducing conditions.
Reducing the pH can also increase the susceptibility to chloride SCC. Figure 5 shows the time to failure for some stainless steels in 40% calcium chloride at 100°C (near neutral redox potential) at two pH values. It can be seen that the threshold stress for 2205 decreases at the lower pH. This is because the potential is moving to the active-passive transition region.
The factors affecting redox potential are interactive, and this must be borne in mind when selecting materials. As an example, some corrosion tests were carried out on S32760 super duplex in a simulated mineral processing fluid. The fluid composition was:
| Chloride | – | 1 to 90 g/L |
| MgSO4 | – | 75 g/L |
| NiSO4 | – | 9.2 g/L |
| CoSO4 | – | 1.0 g/L |
| MnSO4 | – | 2.7 g/L |
| FeCl3 | – | 1.5 g/L |
| H2SO4 | – | 38 g/L |
With the acid the pH was low, and the ferric chloride made the solution oxidising. Autoclave tests were conducted at different temperatures and the results are shown in Table 3. The results show that SCC only occurred at the highest temperature and chloride concentration, while pitting only occurred at the lowest temperature and a relatively high chloride concentration. This is because the type of corrosion depends on exactly where you are on the polarisation curve (Figure 3) under any particular set of conditions.
Reducing conditions
It is also possible to move the potential to the active/passive transition region when reducing chemicals are present. One of the most common is hydrogen sulphide, as found in sour oil and gas wells. Stainless steels can suffer SCC in sour fluids, but only if chloride is also present10. The use of metals in sour oil and gas fluids is specified in ISO 15156/NACE MR017511. For duplex stainless steels the limits of use are 10 kPa partial pressure of H2S for S32205, and 20 kPa for super duplex. However, it is well known that the chloride content and in-situ pH also affect the resistance to SCC. Francis combined the results of SCC tests from a number of laboratories to derive the safe use limits for duplex and super duplex stainless steel, as shown in Figure 67. The results show that the duplex alloys can be used at higher H2S partial pressures than the ISO 15156/NACE MR 0175 limit at lower chloride concentrations, and at higher pH values.
Service experience
There have been numerous successful applications of duplex stainless steels in industry because of their good resistance to SCC. Many of these have been recorded in the proceedings of the numerous duplex stainless steel conferences held since 1991. Table 4 shows some examples from the refinery and petrochemical industries, where duplex alloys have been chosen for their resistance to corrosion and SCC7. There have been a few failures of duplex alloys by SCC under oxidising conditions. Linton et al reported the failure of a super duplex separator vessel in an ammonium nitrate plant12. The environment was 82% ammonium nitrate, 16% water, 0.8% ammonia and 0.8% nitric acid at ~180°C. Only a few mg/L chloride were reported in the fluid, but the nitric acid would make the fluid very oxidising, and the results in Figure 4 suggest that at 180°C even a few mg/L chloride would cause SCC under strongly oxidising conditions.
Super duplex S32550 was used for agitator shafts in the autoclave at a mineral processing plant. The autoclave was pressurised with oxygen at 31 bar at 220°C, and the fluid contained ~ 30g/L sulphuric acid and less than 50 mg/L chloride. Severe cracking occurred in several shafts as shown in Figure 7. As above, the oxygen made the environment highly oxidising, and it would not take much chloride for SCC to initiate.
There have been many examples of the use of duplex stainless steels in sour brines outside the limits in ISO 15156/NACE MR0175, as shown in Figures 6A and 6B, without any SCC failures. Woollin and Maligas report the SCC failure of a super duplex stainless steel in a brine containing 20,000 mg/L chloride and 200 kPa H2S at pH 3.512. This point lies above the boundary curve in Figure 6B at pH 3.5, so the failure is hardly surprising.
In reducing alkaline environments, duplex stainless steels are very resistant to both SCC and corrosion. The Kraft pulp and paper process is a good example, where sodium sulphide is used as a catalyst with the caustic soda. Duplex stainless steels have found a wide variety of uses in Kraft mills7, and Figure 8 shows a S32304 digester and impregnation vessel digester awaiting assembly.
Common species affecting the redox potential
There is a wide range of oxidising and reducing chemicals, but many are rarely found in service environments. Even when present, they need to be at sufficient concentration to have an effect, and the chemical environment must be one where reduction of the oxidising species is possible.
In some processes there is an overpressure of oxygen, which clearly creates oxidising conditions. Some other oxidisers that have been observed to cause SCC are cupric and ferric irons and chromium and vanadium1, all of which are more likely to be present in significant quantities in mineral processing operations. Sulphide is one of the most common reducing agents present in significant quantities in industrial environments.
Alternative alloys
Where lower alloy duplex stainless steels may be susceptible to SCC, the use of a higher alloy duplex may be sufficient to prevent failures. In oxidising environments, titanium and its alloys offer an alternative to stainless steels, albeit at an increased cost, provided that there are no fluorides present. It is important to select the correct grade, as there have been SCC failures of grade 5 titanium in hot, high chloride, low pH oxidising slurries in the mineral processing industry. The high Ni-Cr-Mo alloys (such as UNS N06625 and N10276) are not suitable in highly oxidising environments because in the transpassive region molybdenum salts are soluble and high rates of dissolution occur, and also pitting if chloride is present.
A Ni-Cr-Mo alloy that could be considered is alloy 33 (see Table 1). Its high chromium content gives it an extended passive range, while the high nickel content gives it good resistance to SCC. The low molybdenum content means that it will not suffer excessive dissolution under transpassive conditions. Because of the limited corrosion data for this alloy, it is advisable to test it in the actual service environment or a close laboratory simulation of it.
In reducing environments containing large quantities of H2S at elevated temperatures and low pH values, alloys 825 and 625 (UNS N08825 and N06625) have no limits of use in the solution annealed condition in ISO 15156/NACE MR0175. In more strongly reducing environments, such as hydrochloric acid, the nickel molybdenum alloys (UNS N10665, N10675 and N10629) are recommended.
Conclusions
- Duplex stainless steels have good resistance to SCC, and there are numerous examples in service where they have performed well.
- Under strongly oxidising or reducing conditions duplex stainless steels may suffer SCC.
- The limits of use in reducing sour brines are well established, but the limits of use under strongly oxidising conditions are less well known.
- Only small concentrations of chloride may lead to SCC in strongly oxidising solutions.
- Where duplex alloys are susceptible to SCC it is necessary to consider more expensive titanium or nickel alloys.
References
D R McIntyre and R Francis (eds), Environmental Cracking: Guidelines for Preventing Stress-Corrosion Cracking, Hydrogen Assisted Cracking and Liquid Metal Embrittlement in the Chemical Process Industries, MTI, St. Louis, MO, USA, 2016
ASTM G36, Standard Practice for Evaluating stress Corrosion Cracking Resistance of Metals and Alloys in a Boiling Magnesium Chloride Solution, ASTM, West Conshohocken, PA, USA.
ASTM G123, Standard Test Method for Evaluating Stress Corrosion Cracking of Stainless Alloys with Different Nickel Content in Boiling Acidified Sodium Chloride Solution, ASTM, West Conshohocken, PA, USA.
R F A Pettersson and E Johansson, Stress Corrosion Resistance of Duplex Grades, Duplex Stainless Steels 2010 conference, Beaune, France, October 2010, KCI.
J Sedriks, Corrosion of Stainless Steels, J Wiley and Sons, 2nd Edition, 1996.
B Ozturk, J J Dunn, J F Grubb, Corrosion Properties of Duplex Stainless UNS S32003 Alloy, Paper 186, corrosion 2008, NACE International, Houston, TX, USA, March 2008.
R Francis, The Corrosion of Duplex Stainless Steels; A Practical Guide for Engineers, NACE International, Houston, TX, USA, 2018.
J D Atkinson, J R Saithala and H S Ubhi, Stress Corrosion Cracking Potentials of Stainless Steels in Dilute Chloride Solutions at 130°C, Eurocorr 2008, Edinburgh, Scotland, September, 2008, European Federation of Corrosion.
R Francis, Galvanic Corrosion: A Practical Guide for Engineers, 2nd edition, NACE International, Houston, TX, USA, 2017.
R Cottis and R Newman, Stress Corrosion Cracking Resistance of Duplex Stainless Steels, Offshore Technology Report OTH 94 440, UK Health and Safety Executive, 1994.
ISO 15156/ NACE MR0175, Petroleum and Natural Gas Industries, Materials for use in H2S – Containing Environments in Oil and Gas Production, Part 3 Corrosion Resistant Alloys, Published by ISO and NACE International.
V Linton, N Laycock, D Keen and P Boon, SCC Failure of a Super Duplex Separator Vessel in an Ammonium Nitrate Plant, Mat. Perf. 47, 9 (2008) 64
P Woollin and M Maligas, Testing of Super Duplex Stainless Steel for Sour Service, Paper 132, Corrosion 2003, March 2003, NACE International, Houston, TX, USA.







![Figure 5. Threshold stress versus time to failure in 40% calcium chloride at 100°C and different pH values [A = pH 6.5; B = pH 1.5] Figure 5. Threshold stress versus time to failure in 40% calcium chloride at 100°C and different pH values [A = pH 6.5; B = pH 1.5]](https://stainless-steel-world.net/wp-content/uploads/sites/3/2023/05/ssw202304-dss-06.jpg)
![Figure 6. Effect of pH and chloride on the SCC resistance of duplex stainless steels7. [A = 2205; B = super duplex] Figure 6. Effect of pH and chloride on the SCC resistance of duplex stainless steels7. [A = 2205; B = super duplex]](https://stainless-steel-world.net/wp-content/uploads/sites/3/2023/05/ssw202304-dss-09.jpg)

![Figure 8. A S32304 digester and impregnation vessel digester for a Canadian Kraft pulp mill awaiting assembly. [Photo courtesy of Steve Clarke] Figure 8. A S32304 digester and impregnation vessel digester for a Canadian Kraft pulp mill awaiting assembly. [Photo courtesy of Steve Clarke]](https://stainless-steel-world.net/wp-content/uploads/sites/3/2023/05/ssw202304-dss-11-1.jpg)