Alloy steels and castings account for around 9% of nickel production and provide specific characteristics for specialised and often critical applications.
By Geir Moe, P.Eng, Nickel Institute
One such material is Ni-Hard. Ni-Hard is a generic name for a family of white cast irons, alloyed with nickel and chromium, suitable for low impact, sliding abrasion for both wet and dry applications.
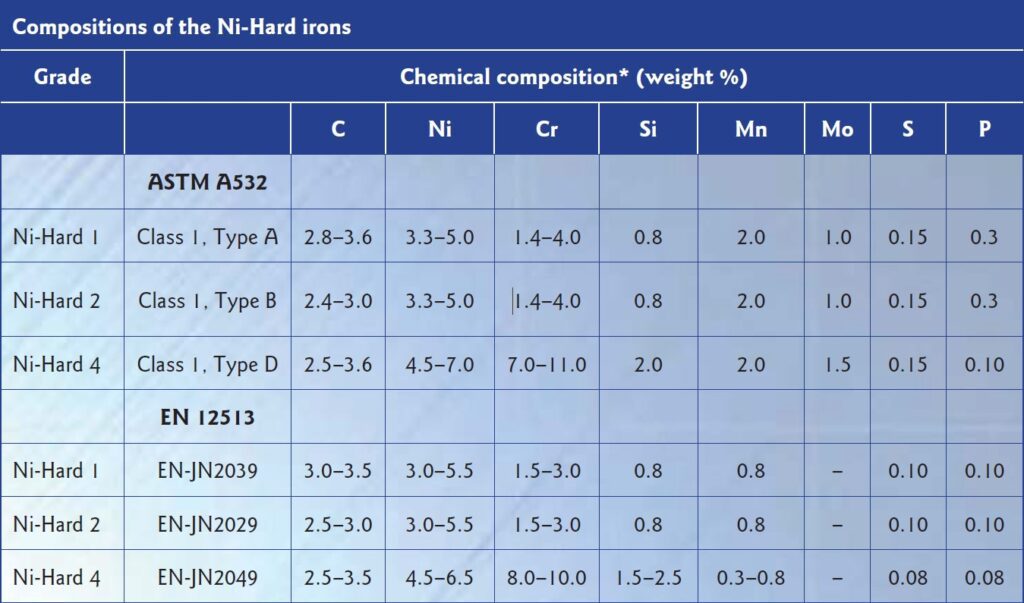
There are three types of Ni-Hard: Type 1, Type 2 and Type 4. Each has slight variations in composition between ASTM and EN specifications, shown in the table below
Nickel is vital in optimising hardness and thus wear resistance. Nickel content increases with section size or cooling time of the casting to inhibit pearlitic transformation. For castings of 38–50 mm (1½–2”) thick, 3.4% to 4.2% nickel is sufficient to suppress pearlite formation which forms as the casting mold cools. Heavier sections may require nickel levels up to 5.5% to avoid the formation of pearlite. It is important to limit nickel content to the level needed to control pearlite as excess nickel increases the amount of retained austenite and lowers hardness.
Pearlite formation

Ferrite is the atomic structure of mild steels that exists below ~727 °C (1,340 °F), while austenite is the atomic structure of steel that exists above this temperature. Austenite is a nonmagnetic solid solution of iron and carbon, while ferrite is magnetic with a lower carbon solubility than austenite. When steel is cooled below ~727 °C, excess carbon is tied up as an iron and carbon compound known as cementite. Cementite and ferrite form a multilayered structure called pearlite, which is present as ‘islands’ within the ferrite matrix.
Typical applications
Ni-Hard Types 1 and 2:
• Metal-working rolls
• Grinding mill liners
• Pulveriser rings
• Slurry pump parts
• Grinding media
Ni-Hard Type 4:
• Slurry pump parts
• Impact blow bars
About the Nickel Institute
The Nickel Institute is the global association of leading primary nickel producers. Its mission is to promote and support the proper use of nickel in appropriate applications. For information, visit: www.nickelinstitute.org


