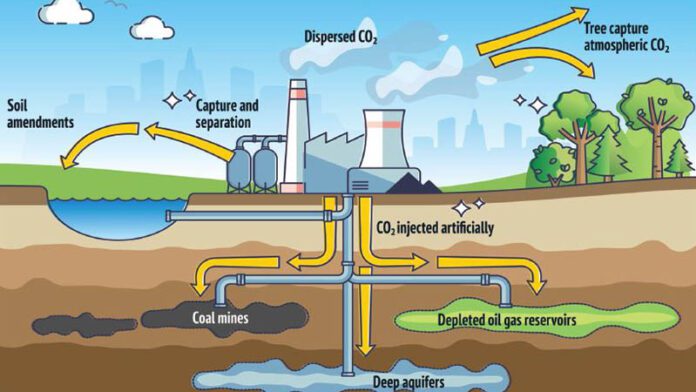
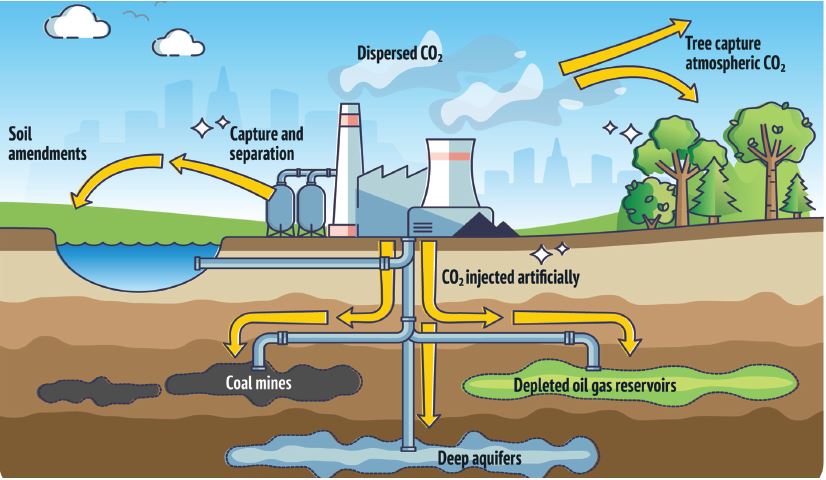
As industries around the globe work to reduce emissions of carbon dioxide, there is also an effort to prevent its escape into the atmosphere by sequestering it. These technologies are known as carbon, capture and storage.
Reprinted with permission from the Nickel Institute
To help achieve the ambition of net zero anthropogenic greenhouse gas emissions, the Nickel Institute continues to explore what kind of contribution and criticality nickel will play in the successful deployment of carbon capture and storage (CCS). This is an endeavour that includes the entire CCS value chain from mature carbon capture, transportation to sub-surface storage.
Going for higher grades
Corrosion and correct material selection are key concerns in the development of safe, reliable, and economical operation of CCS infrastructure. Many CCS processes involve low temperatures with free water present, resulting in acidic conditions and risk of corrosion. As such, carbon steel is not suitable and therefore higher-grade nickel-containing stainless steels and nickel alloys are often required.
Carbon capture from gases often contain water, typically originating from combustion processes. Processes either operate in wet acidic conditions, or require prior drying to capture CO₂. Some operate at high temperatures and in harsh conditions, which are also unsuitable for carbon steel.
Getting there
CO₂ transportation from carbon capture to sub-surface storage will primarily be through pipelines, shipping, trucking and rail. Carbon dioxide is liquefied to enable it to be shipped to a sequestration location. Equipment to transport CO₂ from carbon capture to sub-surface storage also requires nickel-containing low alloy steel, stainless steel or nickel alloys.
Going below
CO₂ injected into sub-surface storage is typically dry and non-corrosive. However, a well must be designed
to account for the risk of acidic conditions being present, leading to corrosion during its lifetime.

Data from well design in the US and EU show that nickel-containing stainless steel and nickel alloys are typically selected for critical well infrastructure at risk of corrosion.
The US has set out clear guidelines for CO₂ injection well design and construction that emphasise selection of materials, supporting the criticality nickel will play in CO₂ sub-surface storage.
To assist in material selection for CCS processes, The Association for Materials Protection and Performance (AMPP) is developing Guidelines for Materials Selection and Corrosion Control for CO₂ Transport and Injection, identifying where nickel-containing materials are preferred. As industries continue to evaluate the value chain of CCS, it is evident there are very few steps that will not need nickel-containing low alloy steel, stainless steel or nickel alloys. This demonstrates nickel’s vital role in deploying CCS to help achieve net zero anthropogenic greenhouse gas emissions in the years and decades to come.
Where is nickel ‘mission-critical’?
– CO₂ recovery from the flue gas of coal-fired power plants, where the flue gas is contaminated with SO₂ and water, creating an acidic condensate that would corrode carbon steel.
– Liquid solvents, such as amine, absorb CO₂ from the gas stream. This can result in acidic corrosive conditions in the CO₂ absorber vessel, in the liquid amine handling system, and in the stripper vessel where cleaned CO₂ is released.
– Solid absorbent recovery systems, such as temperature swing adsorption (TSA), also remove CO₂ from a gas stream by interaction with the sorbent. This process involves humid conditions with temperatures swinging from 40 °C to 100 °C producing acidic corrosive environments. Due to the potential formation of carbonic acid, key areas of risk are: the drying process, where austenitic nickel-containing stainless steel is used; and, the pre-capture blower, where nickel-containing duplex stainless steel is used.
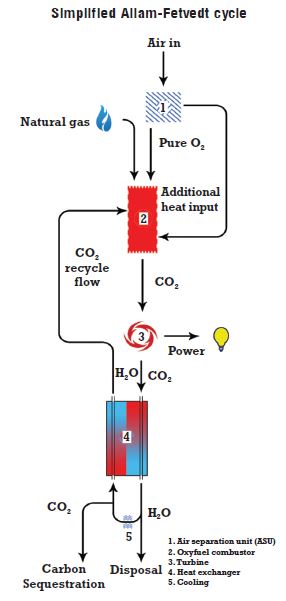
– Innovative processes to utilise and capture CO₂, such as the Allam-Fetvedt power generation cycle, which will require nickel-containing alloys for the CO₂ turbine and combustor, the heat exchanger and the high-temperature piping connecting these two components.
About Nickel institute
The Nickel Institute is the global association of leading primary nickel producers. Its mission is to promote and support the proper use of nickel in appropriate applications. For information visit nickelinstitute.org/